

Across the world, less than 5% of global clinical trials are conducted in Africa, despite the continent carrying a high burden of infectious and non-communicable diseases. This imbalance affects how well treatments reflect genetic diversity, environmental exposure, and local disease patterns.
Our work seeks to close this gap through ethically conducted, community-grounded research.
Clinical trials are systematic investigations involving human participants to evaluate:
Globally, it takes an average of 8–12 years for a medicine to move from discovery to approval. Each phase of clinical trials plays a defined role:
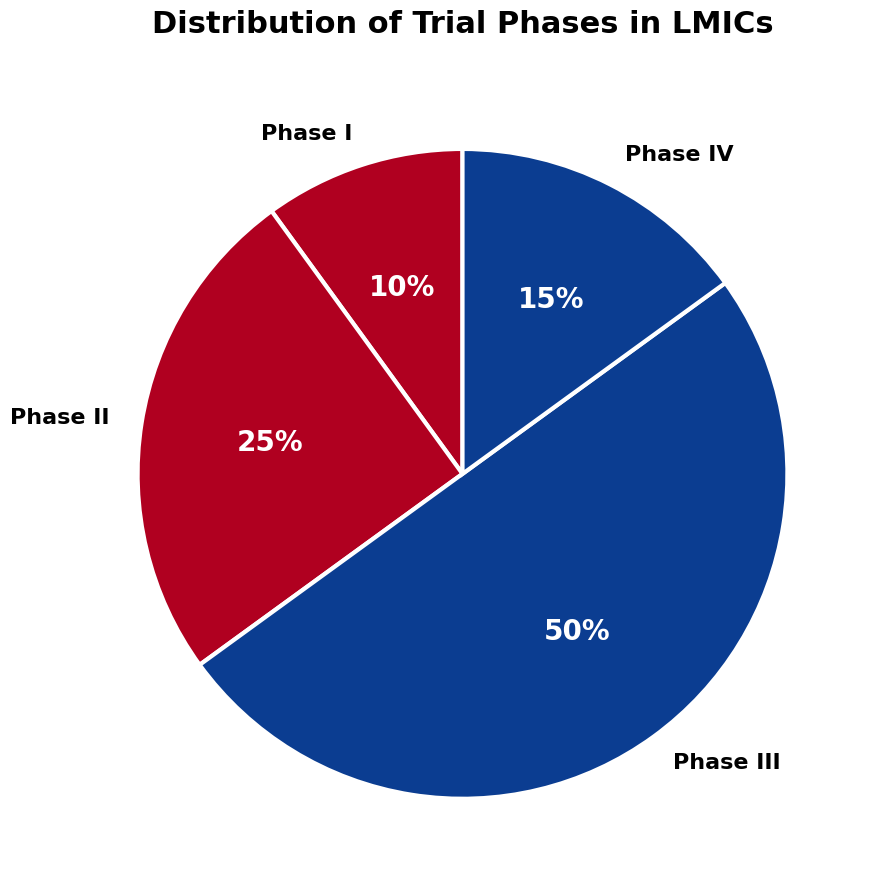
As shown in the chart below, most trials conducted in low- and middle-income countries fall within Phase III. Early-stage research remains limited in these regions, restricting scientific participation and capacity development.
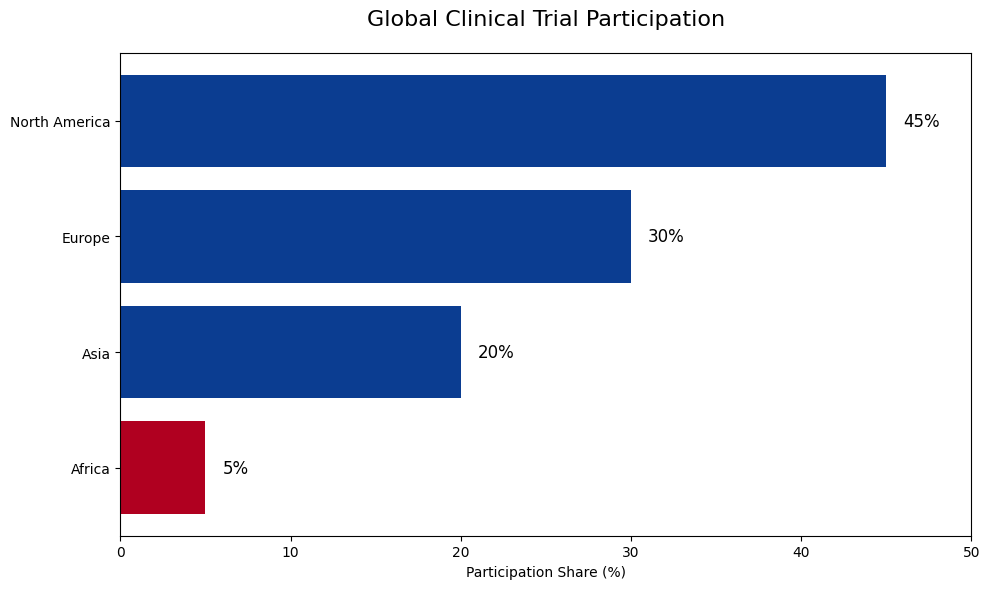
The chart above illustrates the global distribution of clinical trial participation. North America and Europe account for the majority of trial activity, while Africa represents a very small fraction.
This imbalance has consequences:
Given that Sub-Saharan Africa bears nearly 25% of the global disease burden but hosts a small share of trials, expanding ethical research in the region is both a scientific and moral necessity.
Each study is designed around clearly defined protocols, ethical review approval, and strict regulatory compliance.
Participant safety remains central to all research activity. Our clinical trials adhere to:
Community engagement precedes study initiation. Participants are informed in clear language about risks, benefits, and their right to withdraw at any time.
Beyond conducting trials, we invest in:
This strengthens local institutions and contributes to sustainable scientific growth within Nigeria and across Africa. Clinical research is not only about new medicines; it is about equity in scientific knowledge and access to future therapies. Through structured trials, regulatory compliance, and community inclusion, Equitable Medicaid and Clinical Research advances responsible research that reflects the realities of underserved populations.
Expanding Africa’s participation in global trials strengthens global medicine itself.
Questions about our services? Connect with us. Your concerns matter, and we're here to assist you promptly.
Have Questions? Services or Partnership Opportunities
About Us
Our Programs
Useful Links